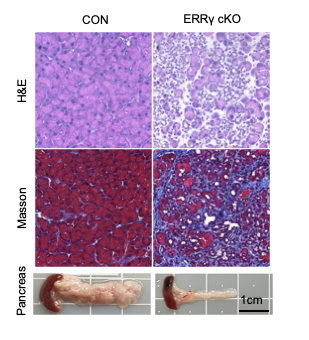
ERRγ deletion causes rapid and progressive pancreatic atrophy
H&E staining (upper panel) and Masson’s trichrome staining (middle panel) of pancreas from control (CON) and ERRγ conditional Knock-out (cKO) cKO mice 7d after the ERR γ deletion.
Gross images of pancreas (lower panel)
We are doing this study to find out why kids with MPS have problems with their bones, joints, brains, and other parts of their body so we can come up with medications that help them.
The Study: This research study collects blood samples from healthy children and adolescents to support research on MPS (Mucopolysaccharidoses), an inherited disease caused by the body’s inability to produce specific enzymes. The missing or insufficient enzyme prevents cells from recycling waste, resulting in cells not working right throughout the body.
The non-invasive delivery device will be for use in respiratory distress syndrome (RDS), a significant cause of neonatal mortality and morbidity
The Lundquist Institute’s investigator Frans Walther, MD, PhD, has received a two-year grant from the Bill & Melinda Gates Foundation in the amount of $471,858.00 to support the development of a synthetic aerosolized lung surfactant and a non-invasive delivery device for use in infant respiratory distress syndrome (RDS), a significant cause of neonatal mortality and morbidity. Newborns experiencing RDS must dramatically increase the effort to take each breath, due to lack of adequate, naturally occurring surfactant production in their lungs.
JDRF Career Development Awards are highly competitive and given to only a few promising junior researchers each year
The Lundquist Institute’s investigator Eiji Yoshihara, Ph.D., has received a five-year $749,995.50 Career Development Award from JDRF (formerly known as Juvenile Diabetes Research Foundation) for his proposed study on how human β cells gain immune evasive function by environmental cues. In pre-clinical humanized models, induction of immune-evasive function in stem cell derived functional human islet-like oganoids has proven to cure diabetes by reducing the risk of graft rejection.
Jody Spillane, Sr. VP for Public Affairs, Spearheads New Program to Introduce Elementary School Students to Science
LOS ANGELES (February 24, 2022) — The Lundquist Institute today has launched an exciting new program that introduces elementary public-school students to science. The new Institute initiative began offering its science-based curriculum to elementary school students in December of 2021 and will extend its workshops through June 2022 and then continue to offer the program every semester going forward.
Research is funded by five-year, $2.29 million grant from the National Institute of Neurological Disorders and Stroke.
LOS ANGELES (February 2, 2022) — The Lundquist Institute’s investigator Lina R. Nih, PhD, and her colleagues at Penn State have received a five-year, $2.29 million grant from the National Institute of Neurological Disorders and Stroke — an institute within the National Institutes of Health — to explore the use of injectable porous biomaterials to target post-stroke immune response and promote new blood vessel and axon formation, processes known as angiogenesis and axonogenesis, respectively, at the site of the stroke.
The Lundquist Institute float in 2022 Rose Parade called “Impositive” to celebrate its 70th Anniversary.
The Lundquist Institute participated in this past weekend’s Tournament of Roses Parade in celebration of its 70th Anniversary. The float’s theme Impositive means having optimism in the face of seemingly impossible odds. Impositivity propels the Institute forward as it continues its long history of making incredible groundbreaking discoveries and innovations that impact the world.
The Lundquist Institute will have a float in the 2022 Rose Parade called “Impositive” to celebrate its 70th Anniversary.
The Lundquist Institute will have a float in the 2022 Rose Parade called “Impositive” to celebrate its 70th Anniversary. The float is sponsored by Impositivity Media and Emmaus Life Sciences and will feature riders whose lives have been saved by groundbreaking research conducted at the Institute. The Lundquist Investigators responsible for the life-saving therapies, Dr. Emil Kakkis and Dr. Yutaka Niihara, will also be riding on the float along with their former patients and Institute PhD students.
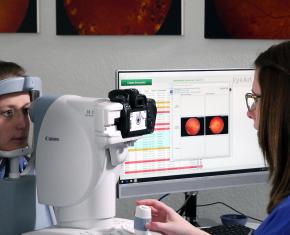
The study examines performance of the EyeArt AI system for detecting eyes with more than mild diabetic retinopathy (mtmDR) and vision-threatening diabetic retinopathy (vtDR).
The Lundquist Institute’s Investigator Eli Ipp, MD, and a multi-center team of investigators have evaluated the performance of the EyeArt AI system in a study published in JAMA Open Network. Dr. Ipp and his co-investigators found that the accuracy of the EyeArt AI system was high in detecting mtmDR (sensitivity 96% and specificity 88%) and vtDR (sensitivity 97% and specificity 90%).
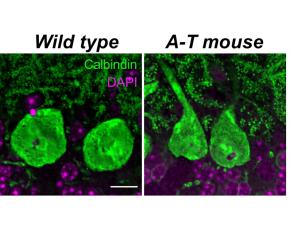
Ataxia Telangiectasia (A-T) Is a Devastating Disorder in Children Characterized by a Severe Loss of Motor Coordination (Ataxia), Increased Cancer Susceptibility, Immunodeficiency, and Premature Death
The Lundquist Institute’s investigators have developed a critically needed mouse model of Ataxia Telangiectasia (A-T), a currently untreatable, rare childhood disorder that results in a severe and progressive loss of motor coordination and premature death by 30 years of age. The disorder is caused by recessive genetic mutations in the A-T mutated (ATM) gene that prevent the production of an important DNA repair pathway protein referred to as ATM.