Leaders from The Lundquist Institute (TLI) participated in Advocacy Day on Sept. 12, 2023, at the nation's capital as part of the Association of Independent Research Institutes (AIRI) annual conference in Washington, D.C. TLI leaders met with congressional staff regarding the importance of federal science research funding. (Pictured from left at the Capitol building: John Munz, Vice President for Human Resources; Kerstin Lynam, Chief Operating Officer; Mike Jones, Chief Financial Officer; Dr. David Meyer, President & CEO; Jody Spillane, Sr.
The Lundquist Institute (TLI) Investigator, Harry Rossiter, PhD, has been awarded a five-year R01 grant totaling $3.8 Million from the National Institutes of Health’s National Heart Lung and Blood Institute (NIH/NHLBI). The R01 is the most prestigious and competitive NIH grant (only 10% are funded) awarded to biomedical researchers.
Dr. Rossiter’s research team at TLI has developed the next generation of the cardiopulmonary exercise test; the standard clinical test to investigate exercise intolerance. The new test, termed muscle-CPET or “mCPET,” will for the first time, integrate direct assessments of neuromuscular performance, with the existing technology that assesses the function of the cardiovascular and pulmonary systems under the stress of exercise.
TRDRP Research grant and JDRF multi-PI grant will help to establish the basis of diabetes and provides new therapeutics
Lundquist Institute investigator Eiji Yoshihara, Ph.D., has received two grants for the research related to type 1 and type 2 diabetes. A three-year $1.45 Million research award from The Tobacco-Related Disease Research Program (TRDRP) and a three-year total $300,000/institution, multi-institutional/multi-PI research grant from the JDRF (formerly known as Juvenile Diabetes Research Foundation). For the TRDRP research, Dr. Yoshihara’s lab and research collaborator Dr.
The research will apply state-of-the-art science to address antibiotic-resistant bloodstream infections caused by Staphylococcus aureus (MRSA) and Candida albicans
The Lundquist Institute (TLI) at Harbor-UCLA Medical Center has announced that TLI Principal Investigator, Michael Yeaman, PhD, has been awarded a grant totaling $11.5M from the National Institute of Allergy and Infectious Diseases (NIAID), Department of Health & Human Services. Along with his role at TLI, Dr. Yeaman is Professor of Medicine at UCLA, and Chief, Division of Molecular Medicine at Harbor-UCLA Medical Center.
The gift will facilitate an FDA-approved multi-site clinical trial under the co-direction of Lundquist Investigators, Dr. Charles S. Grob and Dr. Anthony P. Bossis
The Joe & Sandy Samberg Foundation has made a gift of $300,000 to The Lundquist Institute (TLI) to study the use of psilocybin in alleviating demoralization and the psychological distress associated with life-threatening illness in palliative care. The gift will support the work of Charles Grob, MD, an investigator at TLI and professor at the David Geffen School of Medicine at UCLA and Anthony P. Bossis, PhD, an investigator at TLI. The gift will facilitate an FDA-approved multi-site clinical trial.
The NIH Award will Support Dr. Rehan’s and Advent’s Research to Develop and Position Its Novel Aerosolized Vitamin A Formulation for Commercialization to Prevent BPD
The Lundquist Institute and Advent Therapeutics have announced a landmark collaboration that has resulted in a significant $3 million Small Business Innovation Research (SBIR) Phase IIB grant from the National Institutes of Health (NIH). The funding will directly support the groundbreaking work of Dr. Rehan Virender, which focuses on developing a first-of-its-kind aerosolized vitamin A formulation. This innovative treatment aims to counteract Bronchopulmonary Dysplasia (BPD), a life-threatening condition affecting premature infants, with an eye towards commercialization by 2025.
The Lundquist Institute (TLI) announced that its Institute for Translational Genomics and Populations Sciences contributed to a new study published in Nature Genetics on June 8, 2023 of the DNA of more than 55,000 people worldwide. The study sheds light on how humans maintain healthy blood sugar levels after we have eaten, with implications for our understanding of how the process goes wrong in type 2 diabetes. The findings could help inform future treatments of type 2 diabetes, which affects over 460 million people worldwide and nearly 38 million people in the U.S.
The Lundquist Institute (TLI) announced that its Institute for Translational Genomics and Populations Sciences contributed to a new study published in Nature Genetics on June 8, 2023 of the DNA of more than 55,000 people worldwide. The study sheds light on how humans maintain healthy blood sugar levels after we have eaten, with implications for our understanding of how the process goes wrong in type 2 diabetes. The findings could help inform future treatments of type 2 diabetes, which affects over 460 million people worldwide and nearly 38 million people in the U.S.
Award is for the VX-01 monoclonal antibody (mAb) program targeting the debilitating indication of mucormycosis
The Lundquist Institute (TLI) start-up company, Vitalex Biosciences, has been awarded an SBIR Phase 2 grant from the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health. The grant is for Vitalex’s VX-01, a monoclonal antibody (mAB) program targeting the debilitating indication of the fungal disease, mucormycosis. This serious fungal infection often occurs in people who are immunocompromised and is spreading throughout the world. Mucormycosis is only curable when diagnosed in its early stages.
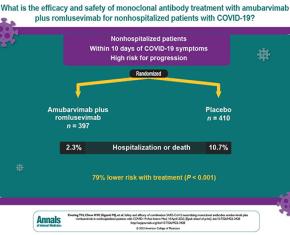
The study showed that antibody combination provides strong protection against severe COVID-19 in large international trial. The trial enrolled non-hospitalized patients with early, mild to moderate COVID-19 who were considered at high risk of progression to severe COVID-19. The participating medical centers were in Argentina, Brazil, Mexico, the Philippines, South Africa, and the United States.
The study showed that antibody combination provides strong protection against severe COVID-19 in large international trial. A treatment combining two antibodies against the coronavirus SARS-CoV-2 strongly protected high-risk people with early COVID-19 symptoms from hospitalization and death in an international Phase 2/3 clinical trial. The trial enrolled more than 800 non-hospitalized patients with COVID-19 at high-risk of progression of the disease in the United States and five other countries.